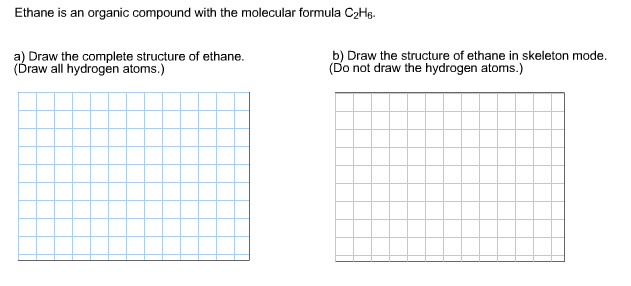
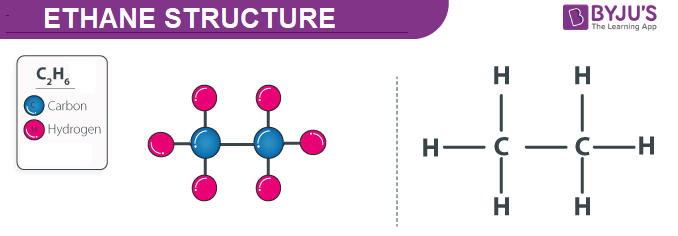
There are two ways to represent the chemical formula of a substance. Ethane is an organic compound with the molecular formula C2H6C2H6.

Ethane Formula Structural And Chemical Formula Of Ethane
B Draw the complete structure of propane.
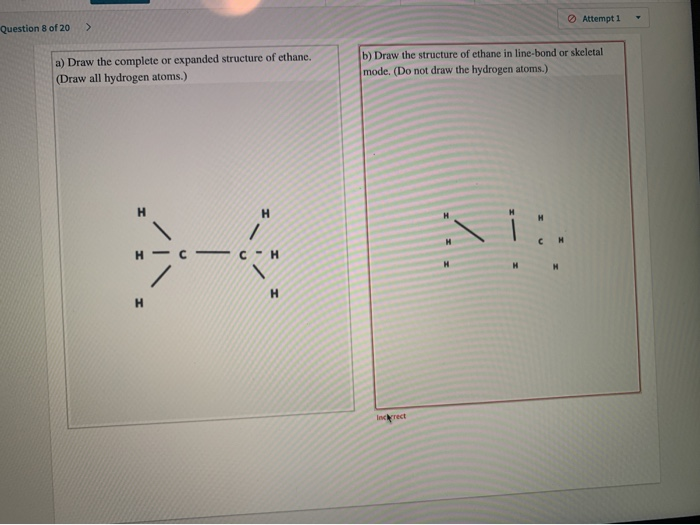
. Draw all hydrogen atoms b Draw the structure of ethane in skeleton mode or as a bondline structure. Ethane has 2 carbon atoms with 6 hydrogen atoms attached to them. Polyethylene glycol divinyl ether.
Its only composed of carbon and hydrogen atoms so it is classified as a hydrocarbon. Do not draw the hydrogen atoms H H H H H н Н H Increct. Composition of ethane molecule.
Draw all hydrogen atoms b Draw the structure of ethane in line-bond or skeletal mode. HCCH Now place one covalent bond between two carbon atoms and place two covalent bonds between carbon and hydrogen atoms. Ethane is an organic compound with a chemical formula of C2H6.
Draw the complete structure of ethane In case you are a enthusiast of nail artwork but usually are not used to the numerous coats of acrylic then this kind of design may well just do the job effectively for you personally. Include all hydrogen atoms. Draw all hydrogen atoms Answer.
The chemical or molecular formula of ethane is-. It is a colorless and odorless molecule that exists as a gas at the standard room temperature. A Draw the complete structure of ethane.
It has a molecular weight of 3007 gmol and is the second simplest organic compound after methane. I ethane ii ethene and iii ethyne. Include all hydrogen atoms.
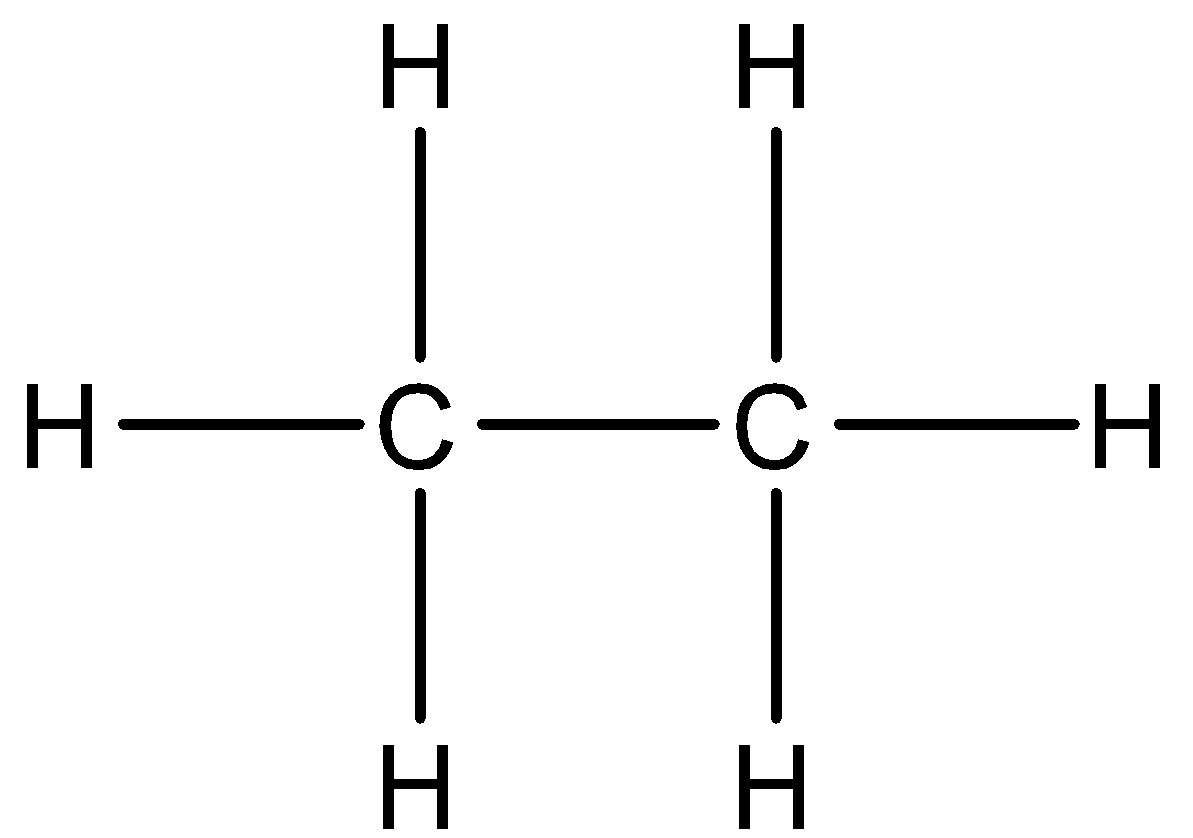
The two carbon atoms are bonded together and three hydrogen atoms are attached to each carbon atom. All the four atoms are placed in one line. Ethane has the chemical formula of C_2H_6 which means that it has two carbon atoms and six hydrogen atoms.
Lewis structures also known as Lewis dot structures or electron dot structures are diagrams that represent the valence electrons of atoms within a molecule. Do not draw the hydrogen atoms Ethane is an organic compound with the molecular formula C2H6C2H6. Ethane is a colourless odourless and flammable gas having two carbon C atoms and six hydrogens H atoms.
I ethane ii ethene and iii ethyne. C2H6 lewis structure. Draw the complete structure of ethane.
Determining the total number of valence electrons in the molecule. ADraw the complete structure of ethane. Skeletal Structure of Ethane.
Draw The Complete Structure Of Ethane. Since the general formula for ethane is CₓH₂ₓ₂ the condensed formula is C₂H₆. Up to 256 cash back a draw the complete structure of ethane.
The molecular formula of the ethane organic compound is C2H6. The suffix ane indicates that compound has no unsaturation so double bond is not present and all the four valency of carbon are satisfied. -When all the lone pairs are placed if the central atom did not complete its octet a double bond should be placed.
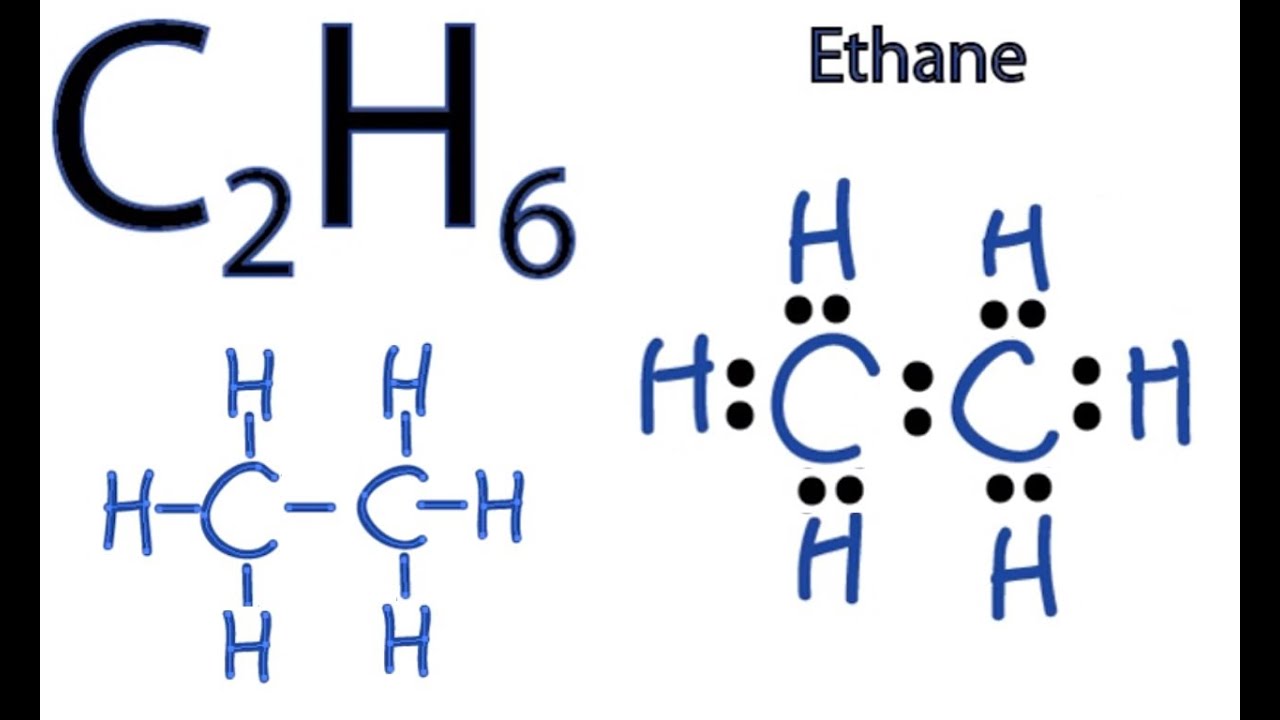
We can draw the electron dot structure of ethane as-Formula of ethane is C_2H_6 In ethane there are two carbon atoms which are singly bonded to each other. In ethane we have two carbon atoms and 6 hydrogen atoms and hence the total number of valence electron are 2 X 4 1 X 6 14. Ethane molecule consists of two carbon atoms and six H-atoms C 2 H 6.
Include all hydrogen atoms Question. In ethane each C-atom is Sp 3-hybridized containing four Sp 3-hybrid orbitalsOne s-orbital and three. Ethane Hybridization Molecular Geometry and shape.
The first step in drawing the Lewis dot structure for ethane C_2H_6 is to determine how many valence electrons are available for the molecule. The complete ethane chemical formula and its structural formula are given in the following points. Let us draw a Lewis structure of ethane step by step.
In condensed form or in structural form. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules whether they exist as lone pairs or within bonds. Ethane is an alkane which consist of two carbon atoms.
Chemistry questions and answers. Include all hydrogen atoms. This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms.
Since C has 4 valence electrons and each H atoms contributes 1 valence electron the total number of electrons will be 24 61 14 e- This means that the Lewis dot structure for C_2H_6 must account for 14. To write the electron dot structure put two hydrogen atoms at terminal positions and two carbon atoms in the internal positions. Attempt 1 Question 8 of 20 a Draw the complete or expanded structure of ethane.
A step-by-step explanation of how to draw the C2H6 Lewis Dot Structure EthaneFor the C2H6 structure use the periodic table to find the total number of val. The valence electron for carbon 1s22s22p2 and hydrogen 1s1 is 4 and 1 respectively. ADraw the complete structure of ethane.
The structural formula or the line-bond structure is sh. A Draw the complete structure of et. Question Write the electron-dot structures for.
Up to 256 cash back Get the detailed answer. B Draw the complete structure of propane. On each carbon atom there are 3 hydrogen atoms.
Ethane is an organic compound with the molecular formula C H. Ethane CH3CH3 or C2H6 CID 6324 - structure chemical names physical and chemical properties classification patents literature biological activities safety. Include all hydrogen atoms.
Write the electron dot structures for.
Illustrated Glossary Of Organic Chemistry Ethane

Ethane Structure Uses Formula Video Lesson Transcript Study Com
Solved Ethane Is An Organic Compound With The Molecular Chegg Com

Ethane Molecular Formula Structure Uses What Is Ethane Video Lesson Transcript Study Com
C2h6 Lewis Structure How To Draw The Dot Structure For C2h6 Youtube
Draw The Structural Formula Of Ethane Draw Electrondot Class 11 Chemistry Cbse
Solved Attempt 1 Question 8 Of 20 A Draw The Complete Or Chegg Com
0 comments
Post a Comment